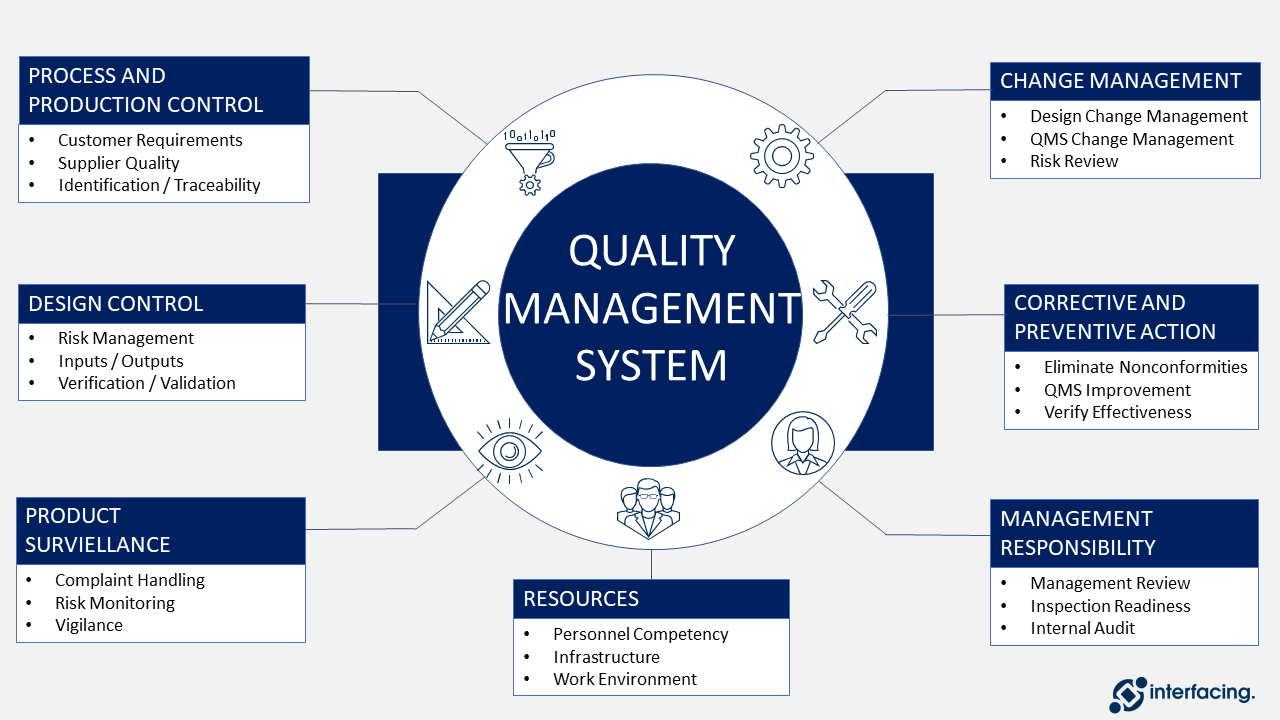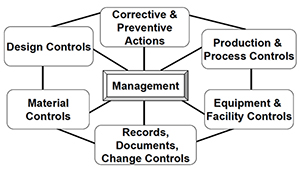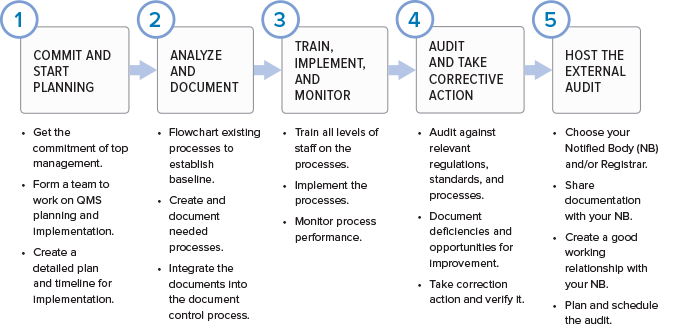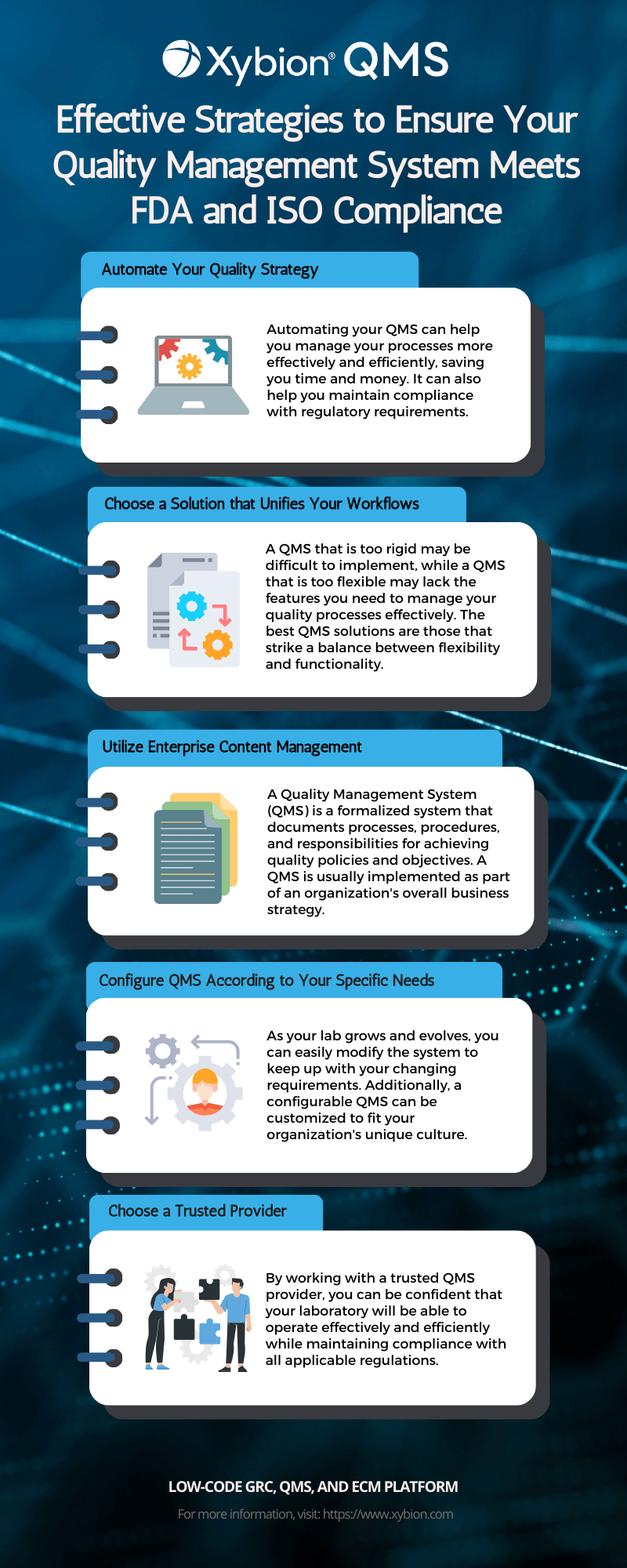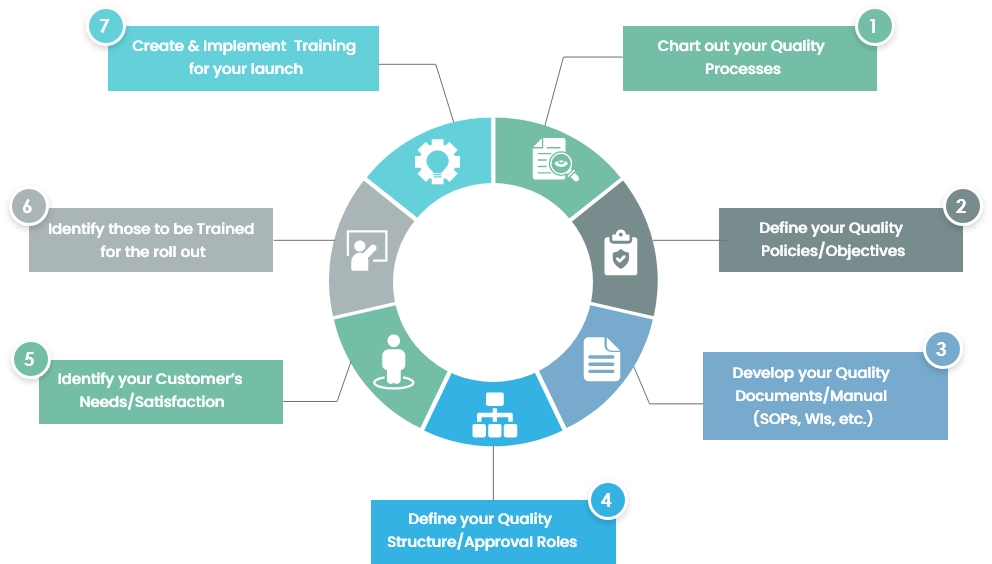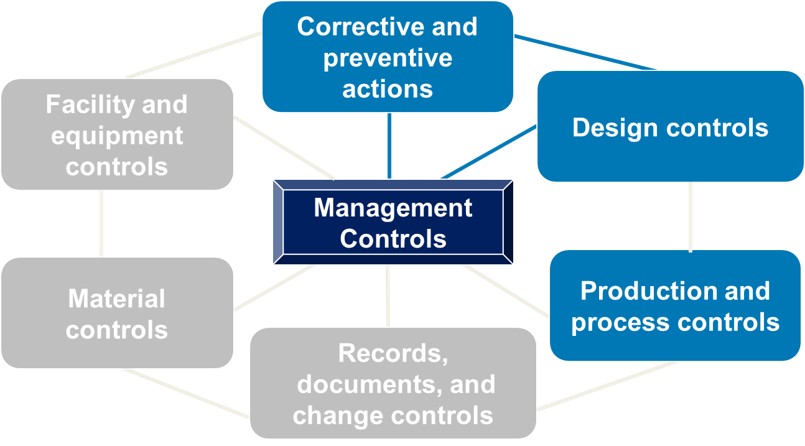
Understanding What Happens During a Medical Device QMS Inspection – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

End-to-End Biologics CDMO Quality Management System|OPM Biosciences – Cell Culture Media and End-to-End CDMO

Designing A World-Class Quality Management System For FDA Regulated Industries: Quality System Requirements (QSR) For cGMP : Muchemu, David: Amazon.fr: Livres
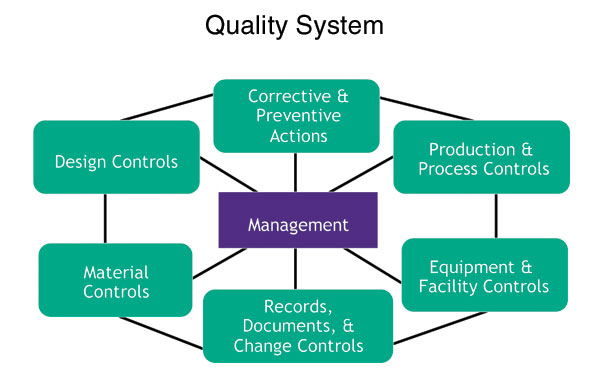
Medical device makers find solution to FDA demands - Tooling and Production Magazine for Large Plant Management and Metalworking